TAR
Total Ankle Replacement in the Literature
Hintermann Series H2™ Literature 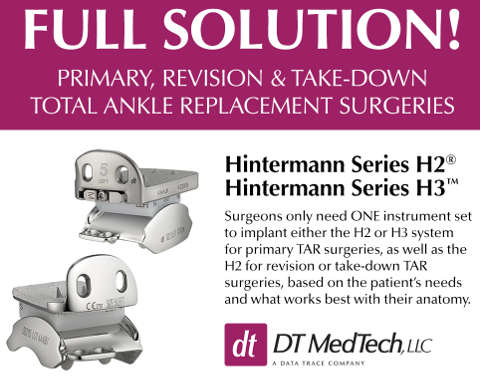
Additional TAR Literature »
Press Releases
FOR IMMEDIATE RELEASE:
DT MedTech Announces the First US Implantation of the Hintermann Series H3™ Total Ankle Replacement System at the Lakewood Ranch Medical Center
September 25, 2019 (Baltimore, MD) – DT MedTech, LLC (DTM) announced today that the first Hintermann Series H3™ Total Ankle Replacement System (H3) has been successfully implanted in a patient in the United States. The surgery was performed, implanting the H3, three-component, non-cemented, non-constrained total ankle replacement system, at Lakewood Ranch Medical Center in Sarasota, Florida by Dr. James Cottom of Florida Orthopedic Foot and Ankle Center.
 The Hintermann Series H3™ Total Ankle Replacement System is indicated for use as a non-cemented implant to replace a painful arthritic ankle joint due to primary osteoarthritis, post-traumatic osteoarthritis, or arthritis secondary to inflammatory disease (e.g., rheumatoid arthritis, hemochromatosis, etc.).
The Hintermann Series H3™ Total Ankle Replacement System is indicated for use as a non-cemented implant to replace a painful arthritic ankle joint due to primary osteoarthritis, post-traumatic osteoarthritis, or arthritis secondary to inflammatory disease (e.g., rheumatoid arthritis, hemochromatosis, etc.).
Dr. Beat Hintermann, world-renowned foot and ankle surgeon, developed both the Hintermann Series H3™ and the Hintermann Series H2® Total Ankle Replacement Systems. Dr. Hintermann stated, “Since the H3 launch outside of the US (OUS) 18 years ago, it has been a goal of mine to also have the H3 available in the US. We reached a huge milestone in June of this year when we received PMA approval. This first surgery means that even more patients will now have access to this device with the positive clinical outcomes it has shown OUS.”
Dr. Cottom notes, “Having implanted hundreds of total ankle replacements, I am excited that we now have the option in the United States to offer our patients either a semi-constrained Hintermann Series H2® (H2) or a non-constrained H3 Total Ankle Replacement System based on the individual patient needs and what will work best with their anatomy. Not all arthritic ankles are the same, so having implant options is very important in achieving successful outcomes.”
David Reicher, President and Chief Executive Officer of DTM, said, “We could not be more pleased to be able to offer this full solution of Total Ankle Replacement Systems to foot and ankle surgeons and their patients. Surgeons only need one instrument set to implant either the H2 or the H3 for primary Total Ankle Replacement surgeries, as well as the H2 for revision or take-down Total Ankle Replacement surgeries. We expect a full US product launch of the H3 in December of this year, while we continue to focus on intense training for our surgeons.”
About DT MedTech, LLC
DT MedTech, LLC is the parent company of DT MedTech International Limited and European Foot Platform, S.A.R.L. DTM and its subsidiary companies maintain offices in Baltimore, Maryland; Dublin, Ireland; Saint-Louis, France; and Liestal, Switzerland. The Data Trace family of businesses has been a leader in scientific and medical publishing, marketing, surgical training, clinical trial management, medical malpractice insurance, and information services for more than 30 years.
Press Releases Archive
- 06/05/2019 FDA Premarket Approval Granted for DT MedTech’s Hintermann Series H3™ Total Ankle Replacement System.
- 02/14/2018 DT MedTech Announces Successful Total Ankle Revision Procedures Using The New Hintermann Series H2™ Tibial Assembly Component and PE Inlay.
- 02/07/2018 DT MedTech Announces Successful Implantations of the New Hintermann Series H2™ Total Ankle Replacement Prosthesis.
- 11/08/2017 DT MedTech Announces 510(k) FDA Clearance for Hintermann Series H2™ Total Ankle Replacement System
- 08/15/2016 DT MedTech Secures CE Marking and begins Active Distribution of Hintermann Series H3™ Lower Extremity Medical Devices
- 04/29/2016 DT MedTech Assumes Production and Distribution of Hintermann Series™ Lower Extremity Medical Devices
- 02/29/2016 DT MedTech Acquires Worldwide Ownership of Hintermann Series of Lower Extremity Replacement and Revision Medical Devices
Triple Arthrodesis in Adults Using Rigid Internal Fixation: An Assessment of Outcome
Foot Ankle Int. 1999 Jun;20(6):356-363 Bednarz PA, Monroe MT, Manoli A 2nd ABSTRACT BACKGROUND: The intermediate outcome of patients who underwent a triple arthrodesis for the treatment of adult foot disorders was evaluated with an outcome tool to determine if their pain and functional status were improved. METHODS: We evaluated 63 … Read more
Triple Arthrodesis of the Tarsus as a Treatment for Posttraumatic Conditions
Acta Orthop Scand. 1966;37(3):328-332 Kivilaakso R, Salenius P ABSTRACT The authors report the results of a follow-up examination of 32 patients in which triple arthrodesis of the tarsus was performed to relieve pain or to correct deformity after different fractures in the ankle region. The most dominant indication for operation was pain … Read more
The Axis of the Ankle Joint and Its Importance in Subtalar Arthrodesis
Acta Orthop Scand. 1963;33:320-328 Wyller T ABSTRACT Barneft, Napier & Hicks showed that the axis of the ankle joint passes obliquely in relation to the sagittal plane of the foot. This means that the foot moves in the ankle joint like a poorly mounted wheel, it swerves from side to side. … Read more
The Effect of Tibiotalar Fixation on Foot Biomechanics
Foot Ankle Int. 1997 Dec;18(12):792-797 Wayne JS, Lawhorn KW, Davis KE, Prakash K, Adelaar RS ABSTRACT BACKGROUND: Contact areas and peak pressures in the posterior facet of the subtalar and the talonavicular joints were measured in cadaver lower limbs for both the normal limb and after fixation of the tibiotalar joint. METHODS: Six … Read more
The Long-Term Results of Ankle Arthrodesis
Acta Orthop Scand. 1981;52(1):107-110 Boobbyer GN ABSTRACT Thirty-seven patients who had ankle arthrodesis carried out from 1-17 years previously were reviewed. The commonest indication in this series was post-traumatic osteoarthritis. Five different methods of fusion were used, the most common being the Charnley compression technique. The incidence of union, the fusion position, … Read more
Surgeon Training and Complications in Total Ankle Arthroplasty
Foot Ankle Int. 2003 Jun;24(6):514-518 Saltzman CL, Amendola A, Anderson R, Coetzee JC, Gall RJ, Haddad SL, Herbst S, Lian G, Sanders RW, Scioli M, Younger AS ABSTRACT BACKGROUND: This study assessed the problems with initial use of ankle arthroplasty by surgeons who were trained by observing the surgeon/inventor (group I), who have … Read more
Talocrural Arthrodesis with Absorbable Screws. 12 Cases Followed for 1 Year
Acta Orthop Scand. 1992;63(2):170-172 Partioa EK, Hirvensaloa E, Partiob E, Pelttaric S, Jukkala-Partioa K, Böstmana O, Hänninenc A, Pertti Törmälä P, Rokkanena P ABSTRACT In 11 patients, 12 arthrodeses of the ankle joint were performed by using absorbable self-reinforced poly-llactide (SR-PLLA) or polyglycolide (SR-PGA) screws. 8 patients had posttraumatic arthrosis, 3 rheumatoid arthritis, and 1 … Read more
Subtalar Arthrodesis for Complications of Intra-Articular Calcaneal Fractures
Foot Ankle Int. 2000 May;21(5):392-9. Flemister AS Jr, Infante AF, Sanders RW, Walling AK. ABSTRACT Eighty six subtalar arthrodeses performed between 1985 and 1996 for complications associated with intra-articular calcaneal fractures were retrospectively evaluated. Patients were divided into three Groups: (I) 59 patients with calcaneal malunions (II) 13 patients with failed open reduction and internal … Read more
Supramalleolar Lateral Closing Wedge Osteotomy for the Treatment of Varus Ankle Arthrosis
Foot Ankle Int. 2007 May;28(5):542-548 Harstall R, Lehmann O, Krause F, Weber M ABSTRACT BACKGROUND: In a 5-year period (1996–2001), the authors performed supramalleolar osteotomies for the correction of distal tibial mechanical malalignment of at least 10° with concomitant pain and with or without radiographic evidence of arthritic changes. METHODS: The method was … Read more

