Jeffrey Watts, DO
Richard D. Guyer, MD
Texas Back Institute and Texas Back Institute Research Foundation
Plano, Texas
Rationale
- The gold standard for symptomatic cervical degenerative disease is fusion. Cervical total disc replacement (CTDR) is intended to remove the degenerated tissues and replace them with motion-preserving implants.
- Many studies have found CTDR to be non-inferior or superior to anterior discectomy and fusion( ACDF).
- CTDR may provide more benefit over fusion surgery if motion at the operative level is preserved, and adjacent-level degeneration lessened or prevented.
Goals of CTDR
- Reduce pain
- Preserve motion and improve function
- Possibly lessen the incidence of adjacent segment degeneration
Device Designs
- Designs currently approved range from two metal endplates with a polyethylene insert between them (ProDisc-C), (Mobi-C), to a ball-and-trough articulation (Prestige), to a polyurethane nucleus and a poly urethane sheath contained by porous coated titanium endplates (Bryan).
- These devices are implanted via a standard Smith Robinson anterior cervical approach, similar to ACDF. Discectomy and preparation of the disc space are similar between the two procedures as well.
Primary Indications for CTDR
- Symptomatic one- or two-level cervical degenerative disc disease that has failed non-operative care including pain medications, activity modification, physical therapy, and injections. Symptoms include neck pain and arm pain. Some clinicians would also include myelopathy from soft disc herniation. Only the Mobi-C has 2 level approval by the FDA.
Primary Contra-indications for CTDR
- Osteoporosis, fracture, instability, infection, tumor. Advanced cervical spondylosis with significant facet disease would preclude arthroplasty
Clinical Outcome CTDR
- Hisey, et al. in the trial comparing Mobi-C TDR to ACDF, reported that TDR is a safe and effective treatment for single-level disc degeneration with less adjacent segment degeneration.
- Davis, et al. found the Mobi-C to be as safe and effective as ACDF for the treatment of degenerative disc disease at 2 contiguous cervical levels.
- Janssen, et al. noted both ProDisc-C and ACDF were effective in reducing neck pain and arm pain and improving and maintaining function and health-related quality of life in patients with single-level cervical degenerative disc disease. They also found no significant differences in the rates of device-related adverse events between the two groups.
- For both CTDR and ACDF, Coric, et al. reported the mean neck disability index( NDI) and VAS pain scores improved significantly in both groups at 6 weeks and remained significantly improved at 24 month after surgery. The ROM in the CTDR group decreased at 3 months but was significantly greater than the preoperative mean at 12 and 24 month follow up. The overall success rate was significantly greater in CTDR compared to ACDF.
- Hisey, et al. found the success rate was similar between CTDR and ACDF at 48 months. The NDI, VAS, and SF-12 scores were significantly improved in early follow up in both groups, and maintained throughout 48 months. Subsequent surgery rate and adjacent-level degeneration were statistically higher for ACDF compared with TDR.
- Vaccaro, et al. reported superiority in overall success of TDR (SECURE) compared to ACDF at 24 months. TDR showed significant improvement in pain and function in terms of NDI, VAS, and SF-36 at 24 months, and the percentage of patients experiencing secondary surgical interventions at the index level were lower for the TDR group compared to the ACDF group.
- Phillips, et al. found, clinical measures including neck and arm VAS, NDI, SF-36, and neurological status, were significantly improved from preoperative baselines in both TDR and ACDF groups at 2 years. They found NDI at 2 years was lower for TDR, and there were no statistical differences between groups in rates of surgery-related adverse events or secondary surgical procedures. TDR patients reported lower dysphasia scores and higher patient satisfaction.
- Heller, et al. showed improvement in all clinical outcome measures at 12- and 24 months postoperatively for both TDR (BRYAN) and ACDF. The TDR group had statistically greater improvement in the primary outcome variables including NDI and a lower percentage of adverse events. There was no statistical difference between the groups with regard to the rate of secondary surgical procedures.
- Mummaneni, et al. compared TDR (PRESTIGE ST) with ACDF. They found greater improvement in NDI with TDR. TDR also had a statistically significant higher rate of neurological success as well as a lower rate of secondary revision surgery and supplemental fixation. TDR had greater improvement in SF-36 physical component as well as relief of neck pain. The TDR group returned to work 16 days earlier than the ACDF group and had a significantly lower rate of adjacent-segment reoperation. There were no cases of TDR implant failure or migration.
- Murrey, et al. compared TDR (ProDisc-C) to ACDF and reported that NDI and SF-36 post-op scores were lower compared to preoperative scores for both groups. VAS neck pain and arm pain scores were also lower for both groups. TDR had higher neurologic success. They found ACDF had a statistically significant higher rate of secondary surgeries. They found that the TDR group had a lower percentage of patients on narcotics and muscle relaxants at 24 months.
Radiographic Outcome (Figs. 1 and 2):
- Studies have shown that most patients retain ROM of 4 degrees or more in flexion/extension at the operative level after CTDR.
- Heterotopic ossification is a known possibility, affecting less than 5% of CTDR. This can affect the ROM of the CTDR, but the clinical significance seems negligible—the result being similar to fusion in ACDF.
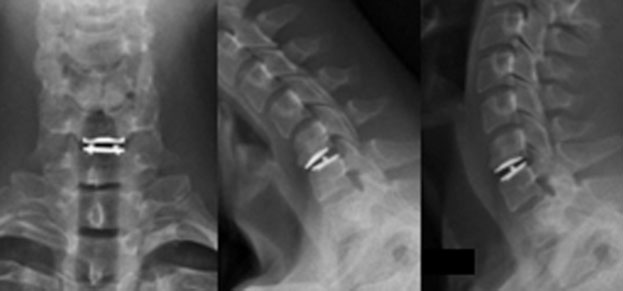
Fig. 1. AP and Lateral radiograhs in flexion and extension of Mobi C TDR at C5-C6
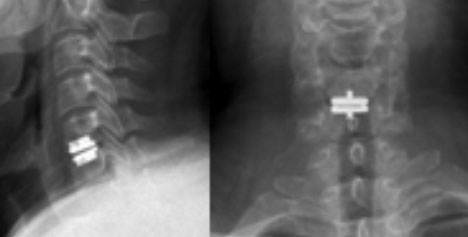
Fig. 2. Lateral and AP radiographs of Pro Disc-CTDR
Complications and Re-operations
- The approach-related complications for CTDR are the same as anterior cervical discectomy and fusion. It includes injury to local neurovascular structures (recurrent laryngeal nerve) and esophageal injury.
- Blumenthal, et al. found that the reoperation rate and time to reoperation for TDR was significantly less than ACDF. They reported that reoperations for adjacent segment changes were less frequent and occurred later in TDR compared with ACDF.
- Coric, et al. found no significant differences between CTDR and ACDF groups when comparing operative time, blood loss, length of hospital stay, or the reoperation rate. They also looked at adjacent-level degeneration between CTDR and ACDF and found more ACDF patients with severe adjacent-level degeneration at 2 years.
- Delamarter, et al. found significantly higher probability of no secondary surgery at the index or adjacent levels at 5 years for CTDR (ProDisc-C) compared to ACDF. They found no ProDisc-C reoperations for implant breakages or device failures. For patients who underwent ACDF, the most common reason for reoperation at the index level was pseudarthrosis. For both ACDF and CTDR, the most common reason for adjacent-level surgery was recurrent neck and/or arm pain.
Postoperative Course
- Postoperative care includes preoperative antibiotic coverage and rehabilitation similar to ACDF surgery with the exception of no concern for fusion in CTDR. Postoperative rehabilitation is left mostly to the surgeon’s discretion but generally may include a soft collar for wound healing for the first 2 weeks. Others may recommend 2 weeks of empiric anti-inflammatories to hypothetically lessen heterotopic bone formation.
Cost
- Cost is always a concern with new treatments.
- Studies have shown both ACDF and CTDR to be cost-effective treatments for cervical DDD.
- Some studies show CTDR to be significantly more cost effective than ACDF which may involve the use of a plate, cage, and biologic substitutes.
- A benefit to CTDR is less cost variability compared to ACDF.
References
- Blumenthal SL, Ohnmeiss DD, Guyer RD, et al. Re-operations in cervical total disc replacement compared with anterior cervical fusion: Results compiled from multiple prospective FDA IDE trials conducted at a single site. Spine 2013;38:1177–1182.
- Coric D, Nunley PD, Guyer RD, et al. Prospective, randomized, multicenter study of cervical arthroplasty: 269 patients from the Kineflex|c artificial disc investigational device exemption study with a minimum 2-year follow-up. J Neurosurg Spine 2011;15:348-358.
- Delamarter RB, Zigler J. Five-year reoperation rates, cervical total disc replacement versus fusion, results of a prospective randomized clinical trial. Spine 2013;38:711-717.
- Heller JG, Sasso RC, Papadopoulos SM, et al. Comparison of bryan cervical disc arthroplasty with anterior cervical decompression and fusion: Clinical and radiographic results of a randomized, controlled, clinical trial. Spine 2009;34:101-107.
- Hisey MS, Bae HW, Davis R, et al. Multi center, prospective, randomized, controlled investigational device exemption clinical trial comparing mobi c® cervical artificial disc to anterior discectomy and fusion in the treatment of symptomatic degenerative disc disease in the cervical spine. Int J Spine Surg 2014;8:
- Hisey MS, Bae HW, Davis RJ, et al. J Spinal Disord Tech 2015;28:E237-243.
- Janssen ME, Zigler JE, Spivak JM, et al. ProDisc-c total disc replacement versus anterior cervical discectomy and fusion for single-level symptomatic cervical disc disease: Seven-year follow-up of the prospective randomized U.S. Food and Drug Administration Investigational Device Exemption study. J Bone Joint Surg Am 2015;97:1738-1747.
- Mummaneni PV, Burkus JK, Haid RW, et al. Clinical and radiographic analysis of cervical disc arthroplasty compared with allograft fusion: A randomized controlled clinical trial. J Neurosurg Spine 2007;6:198-209.
- Murrey D, Janssen M, Delamarter R, et al. Results of the prospective, randomized, controlled multicenter Food and Drug Administration investigational device exemption study of the ProDisc-c total disc replacement versus anterior discectomy and fusion for the treatment of 1-level symptomatic cervical disc disease. Spine J 2009;9:275-286.
- Phillips FM, Lee JY, Geisler FH, et al. A prospective, randomized, controlled clinical investigation comparing PCM cervical disc arthroplasty with anterior cervical discectomy and fusion. 2-year results from the us FDA IDE clinical trial. Spine 2013;38:E907-918.
- Vaccaro A, Beutler W, Peppelman W, et al. Clinical outcomes with selectively constrained secure-c cervical disc arthroplasty: Two-year results from a prospective, randomized, controlled, multicenter Investigational Device Exemption study. Spine 2013;38:2227-2239.
- Patwardhan AG, Havey RM, Khayatzadeh S, et al. Postural consequences of cervical sagittal imbalance: A novel laboratory model. Spine 2015;40:783-92.
- Zhang X, Zhang X, Chen C, et al. Randomized, controlled, multicenter, clinical trial comparing bryan cervical disc arthroplasty with anterior cervical decompression and fusion in China. Spine 2012;37:433–438.
- Grob D, Porchet F, Kleinstuck FS, et al. A comparison of outcomes of cervical disc arthroplasty and fusion in everyday clinical practice: Surgical and methodological aspects. Eur Spine J 2010;19:297-306.
- Park JH, Roh KH, Cho JY, et al. Comparative analysis of cervical arthroplasty using mobi-c(r) and anterior cervical discectomy and fusion using the solis(r) -cage. J Korean Neurosurg Soc 2008;44:217-221.
- Aghayev E, Barlocher C, Sgier F, et al. Five-year results of cervical disc prostheses in the swissspine registry. Eur Spine J 2013;22:1723-1730.
- Davis RJ, Kim KD, Hisey MS, et al. Cervical total disc replacement with the Mobi-C cervical artificial disc compared with anterior discectomy and fusion for treatment of 2-level symptomatic degenerative disc disease: A prospective, randomized, controlled multicenter clinical trial. J Neurosurg Spine 2013;19:532-545.
- Jawahar A, Cavanaugh DA, Kerr EJ, 3rd, et al. Total disc arthroplasty does not affect the incidence of adjacent segment degeneration in cervical spine: Results of 93 patients in three prospective randomized clinical trials. Spine J 2010;10:1043-1048.
- Qureshi SA, McAnany S, Goz V, et al. Cost-effectiveness analysis: Comparing single-level cervical disc replacement and single-level anterior cervical discectomy and fusion. J Neurosurg Spine 2013;19:546-554.
- Bhadra AK, Raman AS, Casey AT, et al. Single-level cervical radiculopathy: Clinical outcome and cost-effectiveness of four techniques of anterior cervical discectomy and fusion and disc arthroplasty. Eur Spine J 2009;18:232-237.
- McAnany SJ, Overley S, Baird EO, et al. The 5-year cost-effectiveness of anterior cervical discectomy and fusion and cervical disc replacement: A Markov analysis. Spine 2014;39:1924-1933.
- Radcliff K, Zigler J, Zigler J. Costs of cervical disc replacement versus anterior cervical discectomy and fusion for treatment of single-level cervical disc disease: An analysis of the blue health intelligence database for acute and long-term costs and complications. Spine 2015;40:521-529.
- Davis RJ, Nunley PD, Kim KD, et al. Two-level total disc replacement with Mobi-C cervical artificial disc versus anterior discectomy and fusion: A prospective, randomized, controlled multicenter clinical trial with 4-year follow-up results. J Neurosurg Spine 2015;22:15-25.

